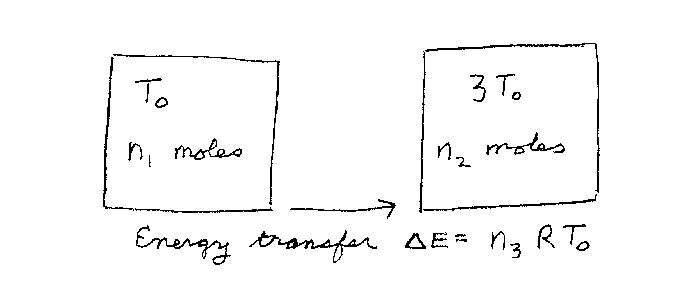
Problem D6:
You have n1 moles of a monatomic ideal gas, which is inititally at
an absolute temperature of T0 K in a container. You also have
n2 moles of the same type of gas at an absolute temperature of
3T0 in another container. You place the two containers in
contact so the gases can exchange energy and they come to thermal equilibrium.
If the amount of energy transfered from one container to the other is
&Delta E = n3 RT0, what is n3? Here R is the
gas constant.
Note that n1, n2 and n3 are unitless.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

