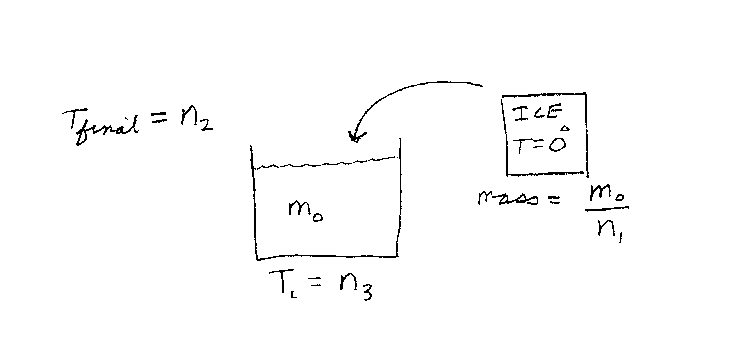
Problem D5:
You have m0 grams of water at an initial temperature of n3 Deg C.
You also have m0/n1 grams of ice at an initial temperature of
0 Deg C. Now, you place the ice into the water. The ice melts and the whole
system becomes water at an equilibrium temperature of n2 Deg C.
What was the initial temperature n3 of the water? Take the specific heat
capacity of water to be 1 cal/Deg, and the latent heat of fusion of ice to be
80 cal. Note that n2 and n3 are in units of Deg C, and n1
is unitless.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

