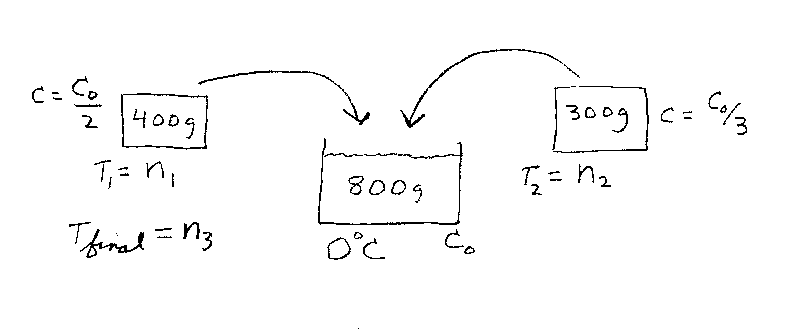
Problem D4:
You have 800 grams of a fluid that is initially at a temperature of 0 Deg C.
The specific heat capacity of the fluid is C0. You have 400 grams of
a solid that is initially at a temperature of n1 Deg C. The specific heat
capacity of this solid is C0/2. You have 300 grams of a second solid
that is initially at a temperature of n2 Deg C. The specific heat
capacity of this solid is C0/3. You now place both solids
into the liquid and the system comes to thermal equilibrium at a temperature of
n3. What is n3?
Note that n1, n2, and n3 are all in the same
temperature units of Deg C.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

