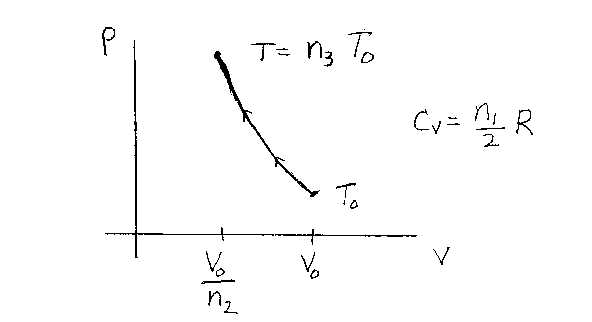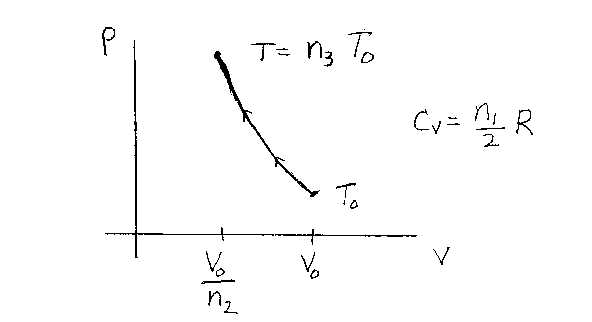Problem D17:
An ideal gas starts out initially at a volume V0 and a temperature
T0. It is then compressed adiabatically to a volume
V0/n2. The molar heat capacity of the gas for a constant
volume process is CV = n1R/2 for the temperatures in
this problem. If the final temperature of the gas is T=n3T0,
what is n3? Note that n1, n2
and n3 are unitless. Note that n1=3 for a monatomic gas,
5 for a diatomic gas, and 7 for a polyatomic gas in the temperature region of the
problem. R is the gas constant.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".