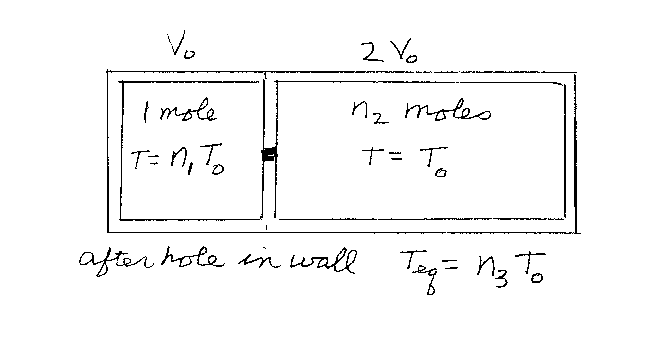
Problem D15:
You have a large container that has two partitions inside separated by
an insulating wall. All the sides of the container are also thermal insulators. The left
side has a volume of V0 and the right side has a volume of
2V0. The left partition contains one mole of a monatomic gas
that is initially at an absolute temperature of n1T0.
The right partition contains n2 moles of the same gas, but at
a temperature of T0. Now someone pokes a hole in the wall between the
two partitions, the gases can mix, and the system comes to thermal equilibrium. If the
final temperature of the system is Tfinal=n3T0,
what is n3? Note that n1, n2, and
n3 are unitless.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

