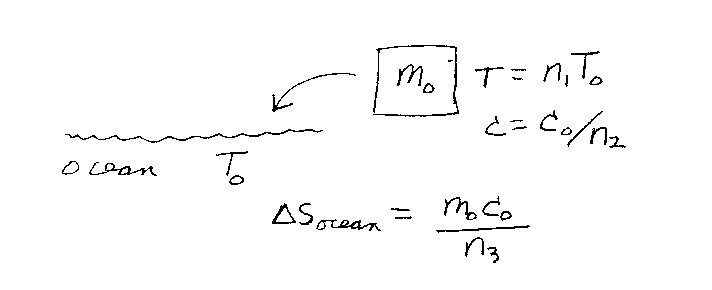
Problem D14:
You have a solid of mass m0 that has an initial absolute temperature of
n1T0. The specific heat capacity of the solid is
c0/n2 where c0 is the specific heat capacity of water.
You throw the solid into the ocean. The absolute temperature of the ocean is
T0, and assume that the ocean's temperature doesn't change when the solid
cools down in the ocean. What is the entropy change of the ocean due to the
solid cooling down to T0. If the change in entropy is
&Delta Socean = m0T0/n3,
what is n3? Note that n1, n2, and
n3 are unitless.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

